Behavioral Neuroscience
Summers
Sensory input for Rhythmicity
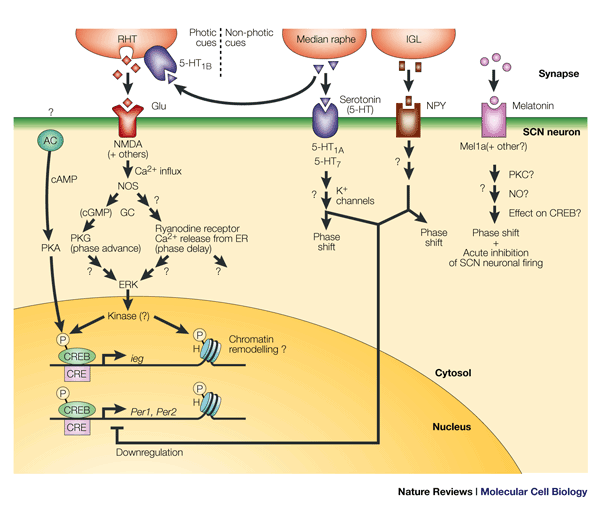
Afferent path to the SCN
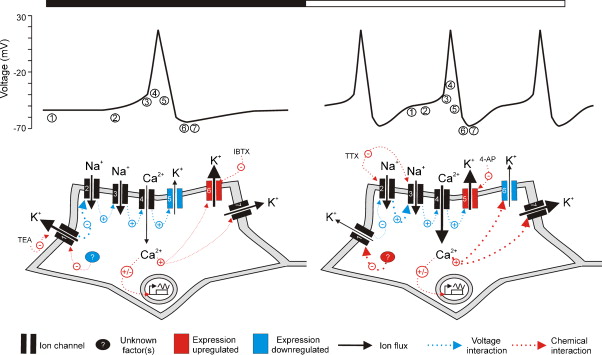
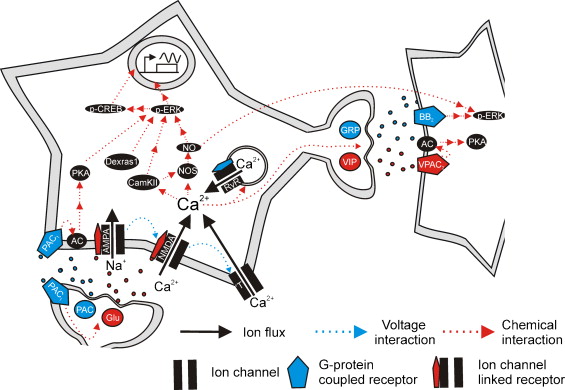
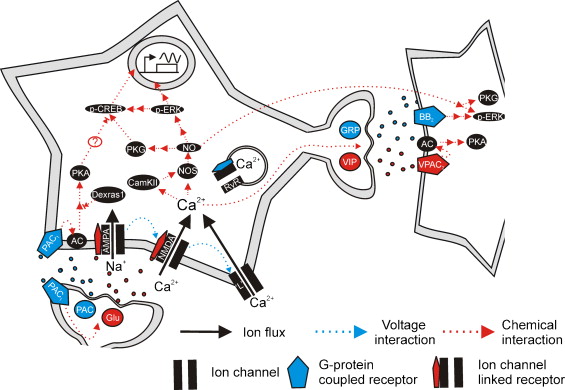
Figures of Rhythmicity
Retina-RGC-SCN
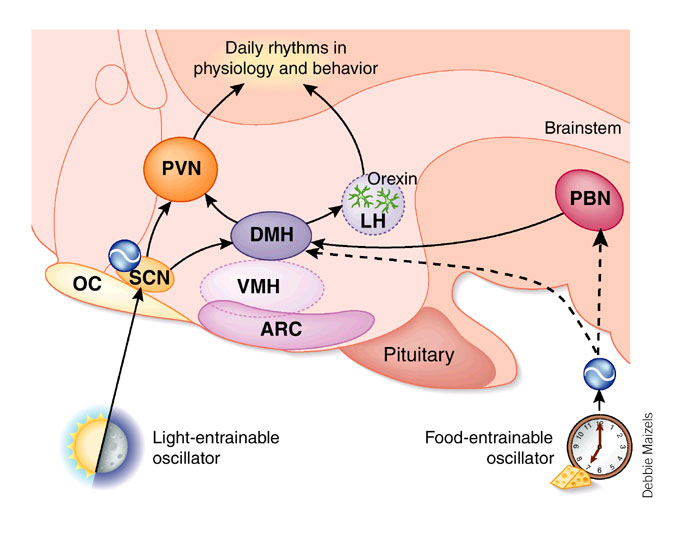
Efferent SCN output
Integration of Rhythms into Behavior
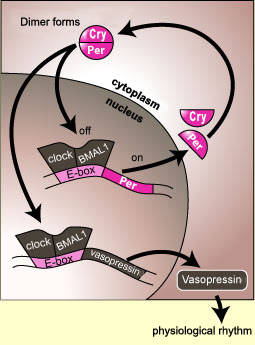
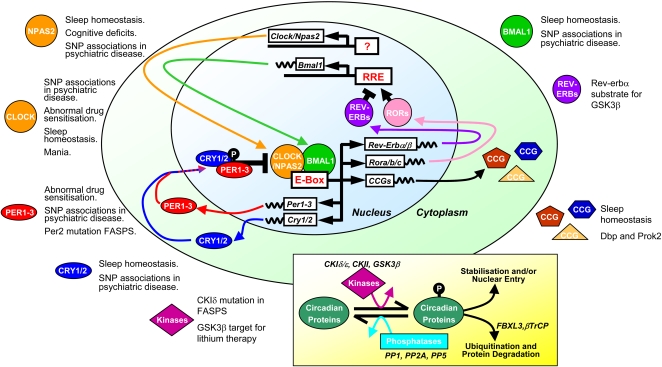
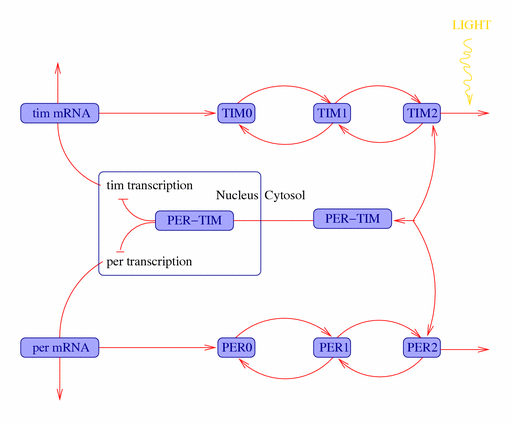
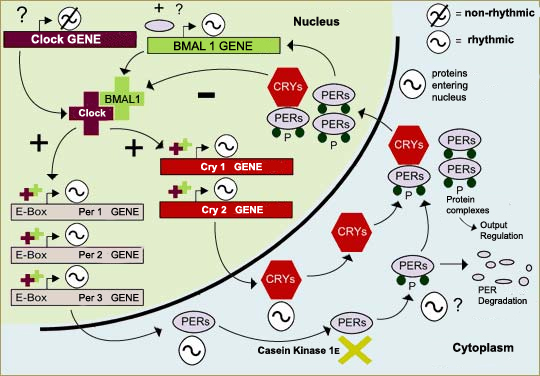
Molecular SCN
end Acronyms/Abbreviations Syllabus
VIP
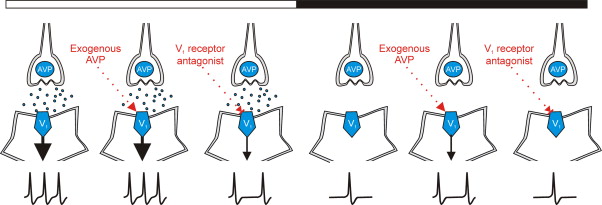
AVP
GABA
5-HT
 the Brain from Top to Bottom
the Brain from Top to Bottom